
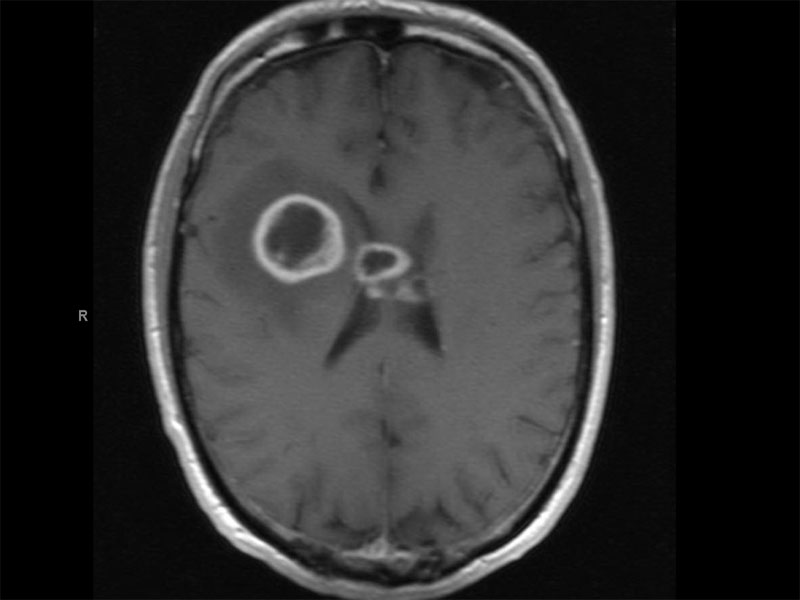
MRI shows a complex mass involving the right frontal lobe, but also involving the corpus callosum and crossing the midlne compatiblw with a high grade glioma. The lesions appear cystic with peripheral enhancing rims compatible with central necrosis. There is mild mass effect
A smear demonstrate cellularity, nuclear pleomorphism and a fibrillary background. Not the pleomorphism is mild to moderate here, it can be quite pronounced with the presence of bizarre multinucleated giant cells.
Case 1 - Serpinginous necrosis is illustrated here, a hallmark feature of GBM. The neoplastic cells line and flank the central geographic necrosis, so-called pseudopalliading necrosis.
The necrosis pattern is often pseudopallisading.
Ki67 labeling demonstrate a brisk mitotic rate, usually exceeding 10-15.
GFAP stains the glial elements (atypical astrocytes).
Case 2 - This case also exhibits extensive necrosis.
High cellularity can be appreciated. The cells are pleomorphic and hyperchromatic.
Proliferating vessels and nuclear pleomorphism are also present.
High EGFR amplification can be surmised from the strong EGFR chromogenic in situ hybridization (CISH). These tumors, along with low levels of B/Akt, tend to respond favorably to therapy.
Astrocytic tumors are divided into two broad categories: diffuse (infiltrative) astrocytic tumors and circumscribed astrocytic tumors. The first group consists of astrocytomas, anaplastic astrocytomas, and glioblastomas. The second group consists of pilocytic astrocytomas, pleomorphic xanthoastrocytomas and subependymal giant cell astrocytomas. The second group has much less malignant potential compared to the first group (Fletcher).
The current universally used WHO grading system for astrocytic tumor is as follows:
- Astrocytoma (WHO II): Tumors with nuclear atypia alone
- Anaplastic Astrocytoma (WHO III): Nuclear atypia with mitotic activity
- Glioblastoma multiforme (WHO Grade IV): Tumors with atypia, mitoses, endothelial proliferation and/or necrosis
Histologically, GBM exhibits marked nuclear and cytologic pleomorphism and tumor heterogeneity (hence, "multiforme"). Cells with dense nuclei and scant cytoplasm as well as bizarre multinucleated giant cells are present. There are also astrocytic cells with delicate fibrillary processes which are PTAH and GFAP positive. Occasionally, epithelial structures (glandular, squamous) may be seen and highlighted by cytokeratin stains. The frequent mitotic activity (some with atypical figures) can be emphasized with Ki67 labeling. Features of glioblastomas that separate them from anaplastic astrocytomas include endothelial proliferation and necrosis.
Endothelial proliferation consists of expansion of cells around the vascular lumina and does not refer to an increase in the number of blood vessels, which is frequently seen in other lower grade gliomas. Note that the proliferative cells are not neoplastic tumor cells, but the normal constituents of blood vessels such as endothelial cells, pericytes and smooth muscle cells (Prayson). It appears that the proliferation is driven by tumor-derived mitogens such as VEGF, thus, the cells lining the vessels become tufted and heaped up i.e. "glomeruloid" (Rosai). The endothelial proliferation tends to occur at the tumor-normal interface. GBM can elicit desmoplasia when it invades the dural and meninges (Fletcher).
GBM can be primary (arising de novo) or secondary (arising from a pre-existing astrocytic neoplasm). The two share identical histologic features, however, they appear to arise via a different molecular pathway.
Secondary GBM demonstrates the following: (1) The most frequent mutation is TP53 (65%), a feature it shares with anaplastic astrocytomas; (2) deletions in p16 (CDKN2a) gene occurs in 19% of cases; (3) EGFR amplication occurs in 8% of cases; (4) PTEN mutations in 4% (Fletcher).
Primary de novo GBM demonstrates the following: (1) TP53 mutation is not common, but mutations in MDM2 has similar affects on the p53/MDM2/p21 regulatory pathway as mutations in TP53; (2) deletions in p16 (CDKN2a) occurs in 31%; (3) EGFR amplifications (40-60%); (4) PTEN mutations (45%) (Fletcher).
Thus, secondary GBM has a lower frequency of p16 deletions, EGFR amplification and PTEN mutations compared to primary GBM. Note that EGFR amplifications are not often seen in pediatric GBMs. LOH at 10q is also seen in 70% of primary GBM and 63% of secondary GBM, thus, is the most prevalent genetic lesion overall (Fletcher).
GBM preferentially affects the cerebral hemispheres in adults and the cerebellum and brainstem in pediatric patients. On imaging, a pushing border and characteristic ring enhancement (corresponding to areas of abnormal vascularization) are seen. Tumor infiltration beyond the macroscopic borders is inevitable and the extent is highly variable. Due to spread along deep fiber tracts, the tumor may cross the corpus callosum to infiltrate the opposite hemisphere, creating a "butterfly" pattern (Fletcher).
Due to extreme tumor heterogeneity, multiple stereotactic biospies are necessary to obtain accurate diagnosis and grading. Standard treatment includes resection of > 95% of the neoplasm, followed by concurrent chemotherapy and radiotherapy.
Once a diabnosis GBM is established, most patients have a survival of 1-2 years. There are attempts to define GBM molecular profiles that may carry an improved prognosis and response to therapy. For example, MGMT promoter hypermethylation appears to be associated with longer time to progression after onset of chemotherapy and longer overall survival (Felsberg).
• Brain : Giant cell glioblastoma
Felsberg J, Rapp M, Loeser S et al. Prognostic significance of molecular markers and extent of resection in primary glioblastoma patients. Clin Cancer Res. 2009 Nov 1;15(21):6683-93. Epub 2009 Oct 27.
Fletcher CDM, ed. Diagnostic Histopathology of Tumors. 3rd Ed. Philadelphia, PA: Elsevier; 2007: 1663-6.
Prayson R, Kleinschmidt-Demasters BK, Cohen ML. Brain Tumors. Consultant Pathology Series New York, NY: Demos Publishing: 2010: 19-21.
Rosai, J. Rosai and Ackerman's Surgical Pathology. 9th Ed. Philadelphia, PA: Elsevier; 2004: 2507-8.