
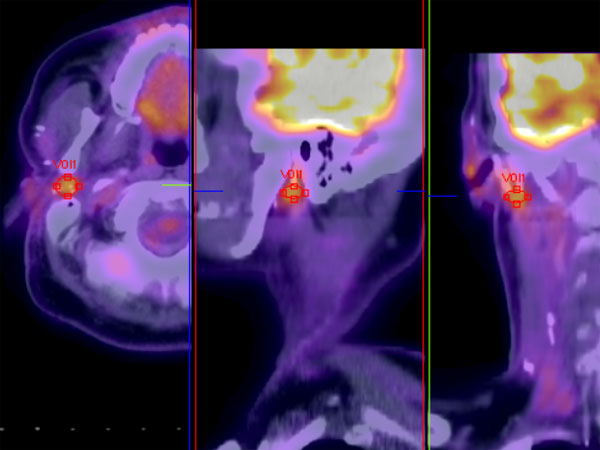
An FDG soft tissue mass centered in the deep lobe of the parotid gland is seen by PET imaging.
The background of the FNA contains numerous apoptotic cells and debris consistent with the lumen of the malignant ducts. Nuclear enlargement is noted within the cell clusters.
The corresponding FNA is extremely cellular and composed of variably sized groups of epithelial cells with a moderate amount of cytoplasm. Pleomorphism is easily appreciated.
The most striking histologic feature of salivary duct carcinoma is its resemblance to ductal carcinoma of the breast. Note the proliferation of neoplastic cells in a cystic pattern with comedonecrosis.
A distinct ductal growth pattern is evident. Oncocytic change (cells containing abundant pink granular cytoplasm) is sometimes quite striking. Mitotic activity is brisk.
This image demonstrates the classic small ducts infiltrating a highly desmoplastic stroma. The stroma is usually strikingly desmoplastic and inflamed, the former being appreciated here. Microscopic variants include sarcomatoid, mucin-rich, and invasive micropapillary types.1,2
Note the prominent stromal fibrosis which is typical of this tumor. This area has a microacinar pattern which resembles acinic cell carcinoma. The remainder of the tumor showed typical salivary duct carcinoma features however.
Yet another example shows a proliferation of oncocytic neoplastic cells.
The cells lining the cyst walls are highly pleomorphic with prominent nucleoli and granular pink cytoplasm. Note the prominent micropapillary architecture. Necrotic cells fill the center of the lumen (left).
Mitotic figures are identified without too much difficulty (arrow).
EMA is often strongly positive in this tumor.
This tumor was mostly negative for her2/neu. A very small focus did show 1-2+ membranous staining, but this likely does not represent true overexpression.
Salivary Duct Carcinoma (SDC) is a highly aggressive neoplasm that resembles ductal carcinoma of the breast. It can occur de novo or as the malignant element of a carcinoma ex pleomorphic adenoma.
Grossly, the tumor is tan, mostly solid and poorly-circumscribed. Microscopically, the defining feature is a resemblance to DCIS or invasive ductal carcinoma of the breast. The intraductal component consists of proliferations of neoplastic cells arranged in a cribriform, papillary or solid patterns with prominent comedo-necrosis. The presence of comedo-necrosis is a key feature!
SDC is immunoreactive for cytokeratin, EMA, and CEA. Most are immunoreactive for p53 and have a high proliferative index with Ki-67. Little or no staining is noted for S-100, myosin, SMA, ER and PR. In terms of more unique stains, 90% are positive for androgen receptor, 80% are positive for GCDFP (gross cystic disease fluid protein-15), 50% are positive for PSA (prostate specific antigen) and 20% are positive for PAP. HER2/neu overexpression, occurring in a third of the tumors, correlates with a more aggressive tumor and poorer prognosis.1,2
Low-grade salivary ductal carcinoma (LG-SDC):
It is important to keep in mind that when pathologists or clinicians employ the term salivary duct carcinoma (SDC), they are referring to 'high-grade SDC' (HG-SDC) described above. There is a separate entity called 'low-grade SDC' (LG-SDC), also known as 'intraductal carcinoma' or 'low-grade cribrifrom cystadenocarcinoma'. This distinction is important because salivary duct carcinoma (HG-SDC) carries a very poor prognosis and warrants aggressive therapy, whereas LG-SDC carries a much more favorable prognosis.
Similar to HG-SDC, LG-SDC affects the elderly and usually involves the parotid gland. However, unlike HG-SDC with a male predominance, LG-SDC affects both genders equally with perhaps a slight female predilection. After complete surgical excision, most patients do very well with no metastasis or deaths from the tumor. Microscopically, LG-SDC resembles atypical ductal hyperplasia or low-grade ductal carcinoma in-situ (DCIS) of the breast. An intact myoepithelial layer must be demonstrated by immunohistochemical stains such as muscle-specific actin. The diagnosis of LG-SDC must be given with caution since it is notoriously difficult to confidently separate in-situ from invasive ductal carcinoma. Thorough sampling and clear communication with the clinician is mandated.1
SDC is an uncommon carcinoma which makes up less than 10% of salivary gland carcinomas, and over 90% develop in parotid gland. Facial nerve dysfunction (perineural spread in 30-60% of cases), intravascular invasion with emboli (31%), pain, cervical lymphadenopathy and rapid growth are some noted characteristics of this tumor.3,4 Male to female ratio is 4:1 -- most cases commonly presenting in the 6th decade of life with advanced disease -- 57% are stage IV on presentation.3
SDC may arise de novo or as a malignant element of carcinoma ex pleomorphic adenoma. In the latter case, the patient may provide a history of a long-standing mass with recent rapid enlargement.
Initial treatment consists of total parotidectomy and cervical lymphadenectomy. As about 60% of patients will present with metastatic disease3 , regional lymphadenectomy is indicated at the time of primary surgery. A weak or paralytic facial nerve preoperatively indicates tumor invasion into this structure, and in this setting, the nerve may need to be sacrificed and grafted to complete an oncologically sound procedure. Removal of the entire gland is important to excise any tumor in the lymph nodes in the deep portion of the parotid or any direct extension to the deep gland.
Post operative radiation therapy to the region is standard treatment as well. Adjuvant hormone therapy may be considered in the future for tumors with hormone receptors.
SDC is one of the most aggressive salivary gland tumors which carries a mortality rate of up to 77% after 3 years. Typically, the recurrence rate of this tumor is high with both regional and distant metastasis frequently seen. For example, the incidence of local recurrence is 35-66%, lymph node metastases (66%) and distant metastases (50-70%).1
Brandwein et al found poor prognostic factors in these tumors were particular glandular location of tumor (submandibular vs parotid), tumor size > 3 cm, infiltrative tumor margins, local recurrence, lymphatic and distant metastases and young age at presentation.6 Her-2/neu over-expression has also been identified as a poor prognostic factor.
• Salivary Gland : Low-Grade Salivary Duct Carcinoma
1 Fletcher CDM, ed. Diagnostic Histopathology of Tumors. 3rd Ed. Philadelphia, PA: Elsevier; 2007: 292-6.
2 Thomspon LDR. Endocrine Pathology: Foundations in Diagnostic Pathology. Philadelphia, PA: Elsevier; 2006: 354-7.
3 Barnes L, Rao U, Krause J, et al (1994). Salivary duct carcinoma. Part I. A clinicopathologic evaluation and DNA image analysis of 13 cases with review of the literature. Oral Surg Oral Med Oral Pathol 78: 64-73.
4 Guzzo M, Di Palma S, Grandi C, Molinari R. Salivary duct carcinoma: clinical characteristics and treatment strategies.Head Neck. 1997 Mar;19(2):126-33.
5 Jeannon JP, Soames JV, Bell H, et al: Immunohistochemical detection of oestrogen and progesterone receptors in salivary tumours. Clin Otolaryngol 24:52, 1999.
6 Brandwein MS, Jagirdar J, Patil J, et al: Salivary duct carcinoma
(cribriform salivary carcinoma of excretory ducts): A clinicopathologic and immunohistochemical study of 12 cases. Cancer 65:2307, 1990.
7 Delgado et al, "Low Grade Salivary Duct Carcinoma - A Distinctive Variant with a Low Grade Histology and a Predominant Intraductal Growth Pattern", Cancer 1996; 78:958-67.