
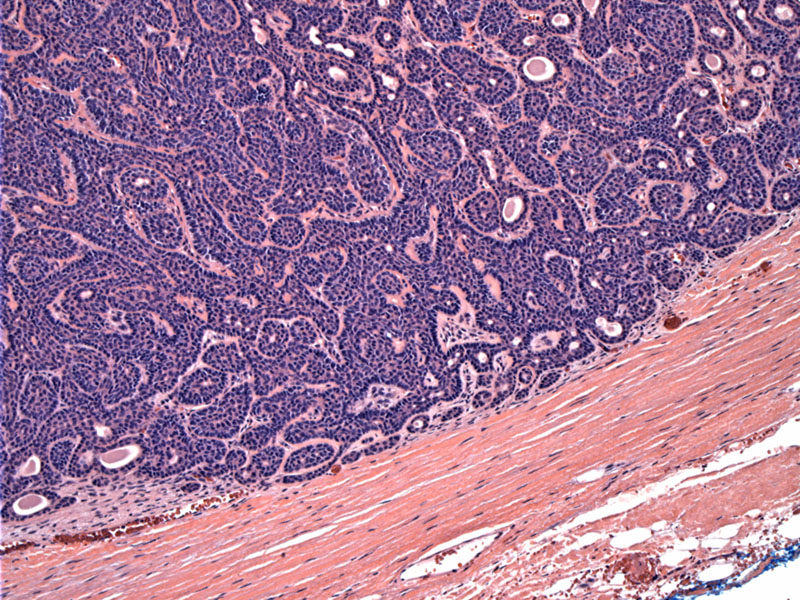
Basal cell adenomas are well-circumscribed. This area shows a fibrous capsule, however, some tumors are not encapsulated. A solid growth pattern of basaloid cells can be appreciated.
Closely packed uniform basaloid cells are arranged in loosely defined anastamosing trabeculae. Basement membrane-like material separates the tumor aggregates from the stroma.
The most peripheral cells show distinct nuclear pallisading. The basement membrane material appears quite abundant and hyalinized, suggestive of the membranous subtype. A PAS stain may help highlight the hyaline material; other features of the membranous subtype must also be considered.
The two cell (luminal and abluminal) population of the basaloid cells can be appreciated here at higher power. The small lumina are lined by cells with slightly more abundant eosinophilic cytoplasm, in contrast to the darker basloid cells in the periphery.
Smooth muscle actin (SMA) stains some areas of the tumor, and has a definite tendency to mark the peripheral cells up again the basement membrane.
EMA is mostly negative in this particular case, but in a few areas there is some luminal staining.
Diff-Quick stain of the aspirated lesion shows a proliferation of monomorphous basaloid cells, without any perceptible architectural features (such as tubules, etc).
The term basal cell adenoma was first proposed in 1967 to describe the benign non-pleomorphic adenomatous tumors of the salivary glands (Kleinsasser). Later in 1970, the basal cell adenoma was combined with the canalicular adenoma under the heading of 'monomorphic adenoma' (Rauch). The most recent WHO classification recognizes basal cell adenoma and canalicular adenoma as separate entities (Seifert).
Grossly, the tumor is well-circumscribed, sometimes with an overlying fibrous capsule. The cut surface is homogenous and tan-brown. Microscopically, the neoplastic cells largely consists of small basaloid cells with dark nuclei and scant cytoplasm. Similar to basal cell carcinoma of the skin, the cells lining the periphery of nests and trabeculae will often palisade. Another population of lighter cells with more abundant cytoplasm will be seen populating the center of islands and nests.
IHC can help highlight this dual luminal-abluminal differentiation with epithelial markers (e.g. CEA, EMA, cytokeratins) highlighting the luminal cells and myoepithelial markers (e.g. calponin, actin) highlighting the peripheral basaloid cells (Fletcher).
There are four morphologic subtypes of this tumor: 1. Solid, 2. Trabecular, 3. Tubular, 4. Membranous. A tumor may display more than one of these growth patterns. Overall, a single pattern will usually predominate. The solid type is most commonly seen.
In contrast to pleomorphic adenomas, basal cell adenomas have a clear demarcation between the cellular component and the stroma. The oft described melting pattern of myoepithelial cells into the stroma characteristic of pleomorphic adenoma is not seen in basal cell adenomas. Furthermore, a chondromyxoid stroma is absent in basal cell adenomas, but a common feature in pleomorphic adenomas.
The malignant counterpart to this tumor, the basal cell adenocarcinoma, can be distinguished from basal cell adenoma by histopathologic findings that include invasive growth patterns, perineural and vascular invasion, tumor necrosis, and increased mitosis (Ellis).
In a study of 11 basal cell adenocarcinomas and 9 basal cell adenomas, the following features favor a diagnosis of basal cell adenocarcinoma: (1) higher proliferative rate as demonstrated by greater than 4 mitotic counts/10 HPF or KI-67 labeling index greater than 5% was seen only in basal cell adenocarcinomas; (2) higher apoptotic index; (3) increased expression of p53 and EGFR; (4) loss of bcl-2 expression. In contrast basal cell adenomas did not express p53 or EGFR and all expressed bcl-2 (Nagao).
Immunohistochemical analysis has been done that allows differentiation of basal cell adenoma from canalicular adenoma.7 These include the strong expression of cytokeratins 7 and 13 in canalicular adenoma and no staining of the solid portion of basal cell adenomas (only when there is tubular differentiation do basal cell adenomas express cytokeratins 7, 8, 14 and 19).
Basal cell adenomas most frequently present in the parotid gland (75% of cases) of patients in the fifth to seventh decades of life. There is a postulated preponderance in women, however, most studies document an even sex distribution (Gonzalez-Garcia). As with most salivary tumors, other sites have been reported in the literature including the minor glands in the oral and nasal mucosa (Mintz). Typically, basal cell adenomas present as a firm, painless, slow growing mass in the superficial portion of the parotid gland.
Of note, the membranous variant of basal cell adenoma has a different clinicohistologic profile. Again, the gender predilection appears to be disputed. Some authors (Thompson) state that males are preferentially affected while others (Fletcher) state there is no gender difference. This variant tends to be multicentric and associated with cutaneous adnexal tumors - so called dermal analogue tumors. Recurrence and malignant transformation are much more common than the other variants.
Surgical excision is the treatment of choice for this tumor.
Excellent with complete excision. Like pleomorphic adenomas, this tumor has the propensity to recur if incompletely excised or if capsular disruption occurs in resection. Not surprisingly, the membranous variant has a much higher rate of recurrence (25%) due to its multifocal nature. Malignant transformation into carcinoma ex monomorphic adenoma (basal cell adenocarcinoma, adenoid cystic carcinoma, salivary duct carcinoma) is approximately 4%. However, the membranous variant has a malignant transformation rate of approximately 28% (Fletcher).
75% occur in the parotid gland.
A monotonous proliferation of cells with darkly staining round nuclei is the characteristic histologic picture
Histologic variants include solid, trabecular, tubular and membranous growth patterns
The membranous variant has distinct clinicopathologic features and is more likely to recur and undergo malignant transformation
• Salivary Gland : Basal Cell Adenoma, Membranous Type
• Brain : Metastatic Ductal Carcinoma of Breast
• Salivary Gland : Pleomorphic Adenoma, Cellular Variant
Ellis GL, Auclair PL. Tumors of the salivary glands. Washington, DC: Armed Forces Institute of Pathology, 1996.
Fletcher CDM, ed. Diagnostic Histopathology of Tumors. 3rd Ed. Philadelphia, PA: Elsevier; 2007: 255-7.
González-García R, Nam-Cha SH, Muñoz-Guerra MF, Gamallo-Amat C. Basal cell adenoma of the parotid gland. Case report and review of the literature. Med Oral Patol Oral Cir Bucal. 2006 Mar 1;11(2):E206-9.
Kleinsasser O, Klein HJ. Basalzelladenome der speicheldrusen. Arch Klin Exp Ohren-Nasen-Kehlkopfheilk 1967;189:302-316
Machado de Sousa SO, Soares de Araújo N, Corrêa L et al. Immunohistochemical aspects of basal cell adenoma and canalicular adenoma of salivary glands. Oral Oncol. 2001 Jun;37(4):365-8
Mintz GA, Abrams AM, Melrose RJ. Monomorphic adenomas of the major and minor salivary glands. Report of twenty-one cases and review of the literature. Oral Surg 1982;53:375-86.
Nagao T, Sugano I, Ishida Y et al. Basal cell adenocarcinoma of the salivary glands: comparison with basal cell adenoma through assessment of cell proliferation, apoptosis, and expression of p53 and bcl-2. Cancer. 1998 Feb 1;82(3):439-47.
Rauch S, Seifert G, Gorlin RJ. Diseases of the salivary glands: tumors. In: Gorlin RJ, Goldman HM eds Thomas Oral Pathology. 6th ed. St Louis, Mo: Mosby–Year Book Inc; 1970:chap 22.
Seifert G, Sobin LH. Histological classification of salivary gland tumors. In: World Health Organization, editor, International histologic classification of tumors. Berlin, Springer Verlag, 1991.