
Mesangial expansion and mesangial cell proliferation are the classic findings in IgA nephropathy.
Another example shows mesangial expansion, but also with some surrounding tubulointerstitial injury.
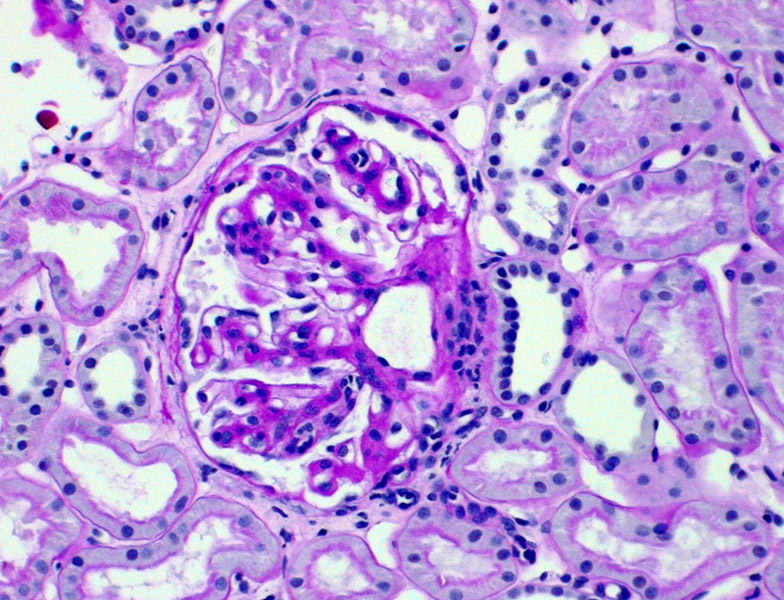
This image demonstrates another injury pattern: fibrous crescent formation and mesangial sclerosis. The background kidney again shows tubulointerstitial injury (tubular atrophy and fibrosis).
Although mesangial cell proliferation and mesangial expansion are the hallmarks in IgA nepropathy, other injury patterns to the glomeruli may also be seen, such as segmental sclerosis (FSGS-like appearance). The areas apart from the sclerotic portion of the glomerulus appear normal.
Immunofluorescence demonstrates non-linear deposits of IgA in the mesangium.
IF of a different patient reveals a similar pattern, with IgA and C3 deposits in a mesangial pattern. Occasionally, a pseudolinear pattern may be seen if the deposits are also distributed in the capillary walls.
Electron microscopy demonstrates mesangial dense deposits.
IgAN is an immune complex glomerulonephritis that involves deposition of dimeric and polymeric forms of IgA1 within the mesangium of the glomerulus. IgA nephropathy (aka Berger disease) and Henoch-Schonlein purpura (HSP) are both characterized by IgA and complement deposition in the mesangium, leading to mesangial injury (proliferation, sclerosis). In addition, HSP has the additional feature of systemic vasculitis, thus, many authorities consider IgA nephropathy to be a variant of HSP in which the pathology is confined to the kidney (Zhou).
IgA nephropathy is the most common glomerular disease worldwide though it exhibits geographic variability. This disease is rarely seen in African Americans.
The prevailing theory of pathogenesis is that IgA nephropathy arises from genetic or acquired immune response to various environmental agents via the respiratory or GI tract, leading to increased IgA synthesis. As you recall, IgA is the main immunoglobulin in mucosal secretions. This theory is supported by the fact that IgA nephropathy occurs more frequently in patients with celiac disease and liver disease. Celiac patients have intestinal mucosal defects and in those with liver disease, there is reduced hepatobiliary clearance of IgA complexes (Kumar).
In its "classic" presentation, IgAN is characterized by a mesangiopathic process with expansion of the mesangial matrix, immunofluorescent staining for IgA, and mesangial cells proliferation. However, the histologic presentation of IgAN is not limited to these findings but also not infrequently include segmental to diffuse endocapillary proliferation. Segmental sclerosis, necrosis with crescentic formation may also be seen. As a reminder, injury patterns of RPGN (rapidly progressive crescentic glomerulonephritis) and FSGS (focal segmental glomerulosclerosis) may be seen in IgA nephropathy. In fact, one of the leading causes of Type II (immune complex) RPGN is IgA nephropathy and likewise, a common cause of secondary FSGS is IgA nephropathy.
Although the aforementioned lesions can raise suspicion for IgA nephropathy, definitive diagnosis can only be made on immunofluorescence. On IF, mesangial deposits of IgA and C3 are seen, and in 1/3 of cases, C1q may be present. On EM, deposits are seen in the mesangium and the perimesangium, beneath the basement membrane as it covers the mesangium.
Patients always present with hematuria, which can be microscopic or macroscopic. The full spectrum of nephritic syndrome, or nephrotic syndrome may be seen in some cases. Rarely, the patient may present with rapidly progressive crescentic glomerulonephritis.
Of note, patients with HSP (usually children) have additional symptoms relating to systemic vasculitis such as palpable pupura, joint pain and abdominal pain (Zhou).
Therapy for IgAN ahould be tailored to the individual clinical and histologic risk factors. For example, patients with hypertension and low-grade (<1.0 g) proteinuria and mild histologic changes such as msangial expansion with or without mesangial hypercellularity), conservative therapy using combination ACE inhibitor and angiotensin receptor blocking therapy is recommended to achieve tight BP control. For those with greater proteinuria, omega-3 fatty supplements can slow progression of renal disease. Patients with accelerated hypertension or nephrotic-range proteinuria or those with proliferative renal lesions may be considered for immunosuppressive therapy.
Cheng L, Bostwick DG, eds. Essentials of Anatomic Pathology. 2nd Ed. Totowa, NJ: Humana Press; 2006: 1122-3.
Kumar V, Abbas AK, Fausto N. Robbins and Cotran Pathologic Basis of Disease. 7th Ed. Philadelphia, PA: Elsevier; 2005: 986-8.
Zhou M, Magi-Galluzzi, C. Genitourinary Pathology: Foundations in Diagnostic Pathology. Philadelphia, PA: Elvesier; 2006: 363-5.