
A direct smear shows dispersed monotonous single cells with densely staining nuclei.
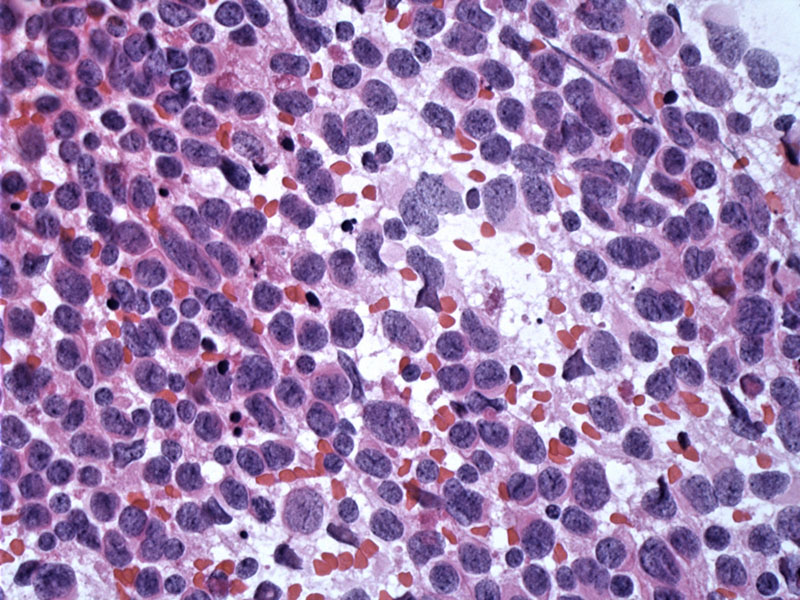
Two distinct populations of cells can be seen here. There are defined round nodules composed of cells with more abundant cytoplasm (neurocytic differentiation), amidst a cellular background of hyperchromatic cells with scant cytoplasm.
Less circumscribed nodules with neurocytic appearing cells are set against a fibrillary background.
The internodular cells are markedly hyperchromatic with little cytoplasm (right) relative to the pinker cells (left).
The border around nodules is usually sharp, but cells with an internodular phenotype can occasionally spill into nodules making the border around nodules less precise.
Separation of the two areas is relatively discrete but can appear focally undulating. Some classic medulloblastomas may show this pattern focally, and the two areas are less well defined and less nodular.
Differentiation along neuronal lines is a feature of the intranodular cells. These areas react for synaptophysin but the internodular areas are negative, the pattern which typifies this variant. Sometimes classic medulloblastoma reacts for this as well but is often negative.
Ki67 appears elevated in the internodular regions and is negative or rare in the nodules, again, another pattern which helps identify this variant.
Reticulin staining is key to differentiating this variant from some morphologically similar areas which may be found in classic medulloblastoma. The desmoplastic variant is defined by its reticulin-positive boundary. By contrast, classic medulloblastoma lacks internodular desmoplasia as evidenced by no reticulin in these areas.
Overall, the most common and most malignant embryonal tumor of the pediatric central nervous system is medulloblastoma. Different histological subtypes of varying neuronal differentiation have been described, and among these desmoplastic medulloblastoma, remains controversial with regards to its impact on treatment response and outcome. The desmoplastic variant has been repeatedly described as indicative of low risk but its significance as a potential predictor of good response to therapy still remains unclear. This variant has been reported to constitute 5%-25% of pediatric medulloblastomas.
Histological recognition of the desmoplastic variant requires identification of a biphasic pattern: (1) nodules (pale islands) of neurocytic areas consisting of larger cells embedded in a more fibrillary neuropil-like matrix. These cells react for neuronal markers (e.g. synatophysin and neurofilament), are less mitotically active and comprise the "reticulin-free" areas; (2) Surrounding these nodules are densely packed cells with a high nuclear:cytoplasmic ratio embedded within a dense, intercellular reticular network. These cells are mitotically active as seen with Ki-67 labeling (Fletcher).
Chromosome 9q loss of heterozygosity (LOH) is exclusively detected in desmoplastic variant and is distinctly lacking in the classic histology medulloblastoma, providing independent evidence that desmoplasia may indeed be pathogenetically distinct (Rieken). One report of desmoplastic medulloblastoma identified an unbalanced t(3;22)(q12;p11.1) chromosomal rearrangement as a sole aberration (Manor).
Gorlin syndrome, also known as Nevoid Basal Cell Carcinoma Syndrome (NBCCS) is an autosomal dominant condition that is not uncommon (1 in 60,000 individuals) and is characterized by multiple basal cell carcinomas, ovarian fibroma and the desmoplastic variant of medulloblastomas. Other features in Gorlin syndrome include facial anomalies, jaw cysts (odontogenic keratocysts), rib malformations, hyperkeratosis of palms and solies. Gorlin syndrome is attributed to a germline mutation of the PTCH1 gene located on chromosome 9q22, which interestingly, is the same aberration seen in both sporadic and syndromic forms of desmoplastic medulloblastomas (Amiashi).
These tumors prefer to arise in the cerebellar vermis in children and as such may cause related symptoms of cerebellar ataxia. Elevate intracranial pressure and hydrocephalus may result from occlusion of the 4th ventricle.
CT scans show a brightly enhancing mass in the cerebellar midline n children, while in adults tumors may arise in the lateral cerebellar areas. The latter may simulate a metastatic lesion in this population.
Radical surgery to a reasonable extent followed by radiotherapy and both combined and adjuvant chemotherapy according to pediatric protocols provide the appears to offer the best results to date (Rieken).
Note, however, that radiation in those with Gorin syndrome can induce invasive BCCs in the radiation field.
All medulloblastomas are WHO Grade IV neoplasms, however, some studies have suggested the desmoplastic variant carries an improved prognosis compared to other medulloblastoma subtypes (suggesting that radiotherapy may not be necessary).
In children younger than three years who received no adjuvant radiotherapy, desmoplastic histology has been associated with PFS as high as 85% after ten years, opposed to classic histology with 34% (Rutkowski). Recurrence of disease may be seen as late as 65 months after completion of initial therapy (Rieken).
• Cerebellum : Medulloblastoma
Amiashi SFA, Riffaud L, Barssier G et al. Nevoid Basal Cell Carcinoma Syndrome: Relation with Desmoplastic Medulloblastoma in Infancy. Cancer 2003;98:618-24.
Rieken S, et al. Outcome and prognostic factors of desmoplastic medulloblastoma treated within a multidisciplinary treatment concept. BMC Cancer. 2010 Aug 23;10:450.
Rutkowski S,et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005 Mar 10;352(10):978-86.
Manor E,et al. Derivative (22)t(3;22)(q12;p11.1) in desmoplastic medulloblastoma. Cancer Genet Cytogenet. 2010 Jan 15;196(2):175-8.